All of the following compounds are soluble in water except – As we delve into the realm of chemistry, we encounter a fundamental principle: the solubility of compounds in water. However, this rule is not without its exceptions. In this exploration, we will unravel the intriguing cases where certain compounds defy the norm and remain insoluble in water.
This discourse will provide a comprehensive understanding of water solubility, examining the factors that govern this phenomenon and exploring the practical implications of these exceptions in various fields.
Water Solubility of Compounds
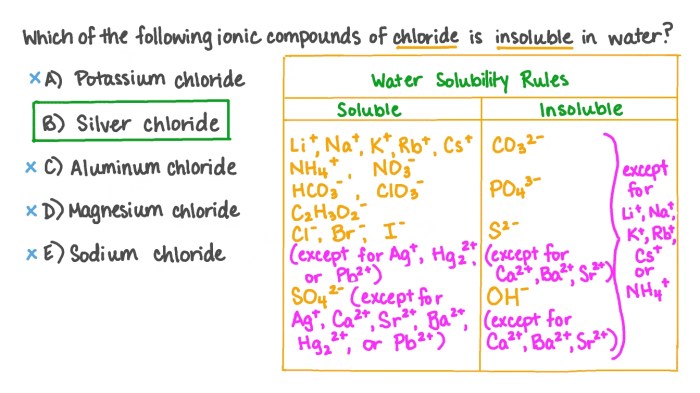
Water solubility is a measure of how well a compound dissolves in water. It is an important property that affects the behavior and applications of a compound.
In general, most ionic compounds and polar covalent compounds are soluble in water. Nonpolar covalent compounds, on the other hand, are typically insoluble in water.
| Compound | Chemical Formula | Solubility in Water | Explanation |
|---|---|---|---|
| Sodium chloride | NaCl | Very soluble | Sodium chloride is an ionic compound that dissolves readily in water. |
| Sugar | C12H22O11 | Very soluble | Sugar is a polar covalent compound that dissolves easily in water. |
| Oil | CnH2n+2 | Insoluble | Oil is a nonpolar covalent compound that does not dissolve in water. |
| Hexane | C6H14 | Insoluble | Hexane is a nonpolar covalent compound that is immiscible with water. |
Exceptions to Water Solubility
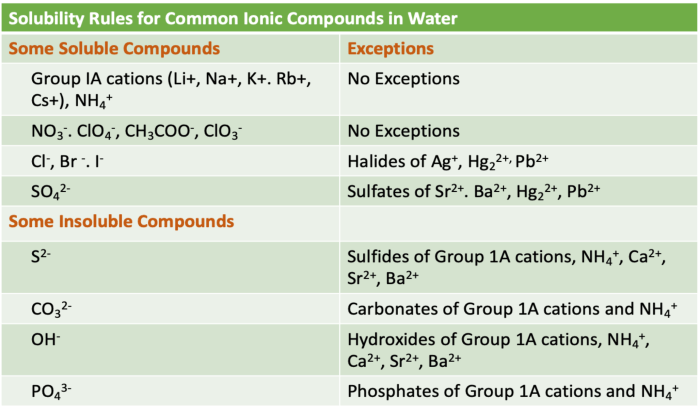
There are a few exceptions to the general rule that most compounds are soluble in water. Some compounds, such as oils and fats, are nonpolar and therefore do not dissolve in water.
Other compounds, such as alcohols and ethers, are polar but have a large hydrocarbon chain that makes them less soluble in water. For example, butanol is a four-carbon alcohol that is only slightly soluble in water, while methanol is a one-carbon alcohol that is very soluble in water.
Factors Affecting Water Solubility
The solubility of a compound in water is affected by several factors, including polarity, molecular size, and temperature.
Polarity is a measure of the uneven distribution of electrons in a molecule. Polar molecules have a positive end and a negative end, which allows them to interact with water molecules. Nonpolar molecules, on the other hand, do not have a permanent dipole moment and therefore do not interact with water molecules as strongly.
Molecular size also affects water solubility. Larger molecules are less soluble in water than smaller molecules because they have a harder time fitting into the water molecule network.
Temperature also affects water solubility. In general, the solubility of a compound increases with increasing temperature. This is because the water molecules become more energetic at higher temperatures and are able to break apart the intermolecular forces that hold the compound together.
Applications of Water Solubility

Water solubility is an important property that has many applications in various fields.
In the pharmaceutical industry, water solubility is important for the development of new drugs. Drugs that are not soluble in water cannot be absorbed by the body and therefore cannot be used to treat diseases.
In the environmental field, water solubility is important for understanding the fate and transport of pollutants. Pollutants that are soluble in water can be easily transported by water and can contaminate drinking water sources.
In the food industry, water solubility is important for the development of new food products. Food products that are not soluble in water cannot be easily mixed with water and therefore cannot be used to make soups, sauces, or other water-based products.
FAQ Insights: All Of The Following Compounds Are Soluble In Water Except
Why are certain compounds insoluble in water?
Solubility in water is influenced by polarity and molecular size. Nonpolar compounds, such as oils and fats, lack the polarity to interact with water molecules, resulting in their insolubility.
What are some real-life applications of water solubility?
Water solubility plays a crucial role in processes like water purification, drug delivery, and extraction of substances from natural sources.